CLINICAL ROUTINE & OPERATIONS
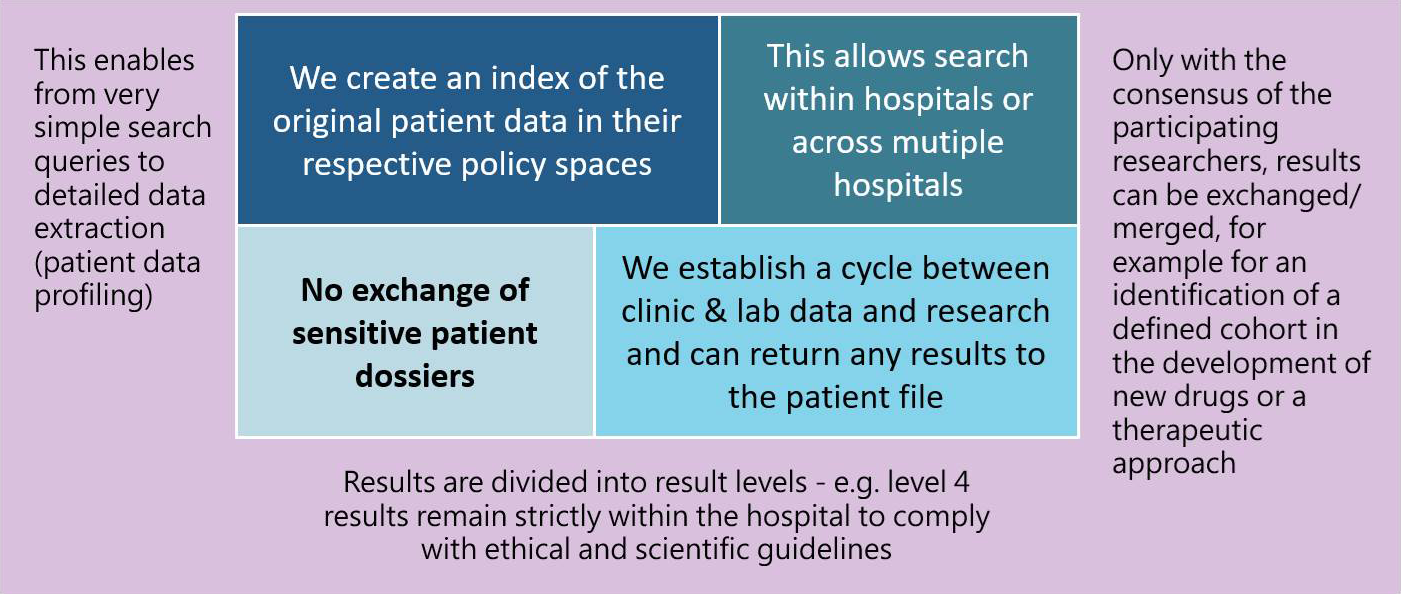
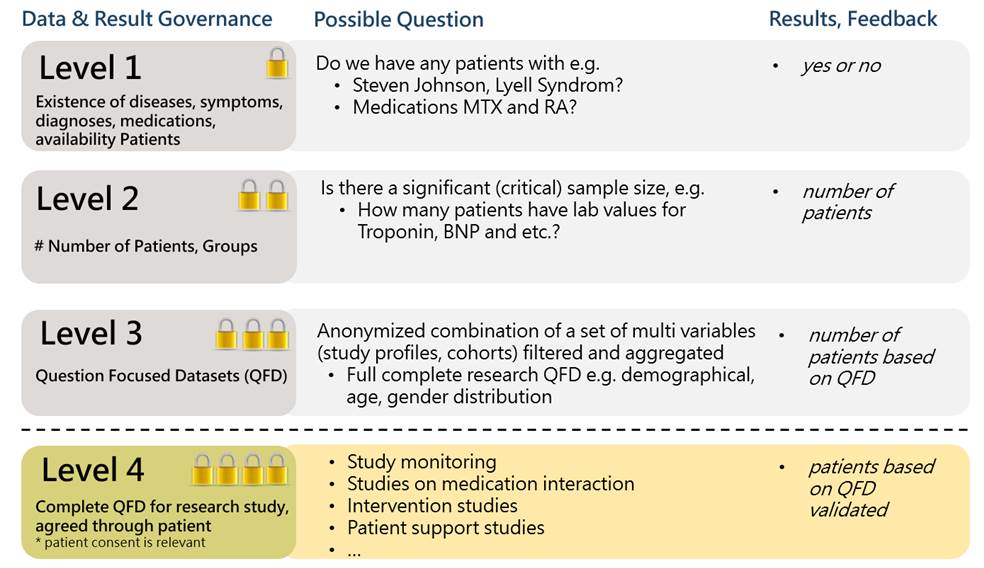
Finding relevant information fast - linking of clinical data, laboratory data and genetic data
Digitalization of Patient Dossier and automation of your Processes
Scanning for Search & View
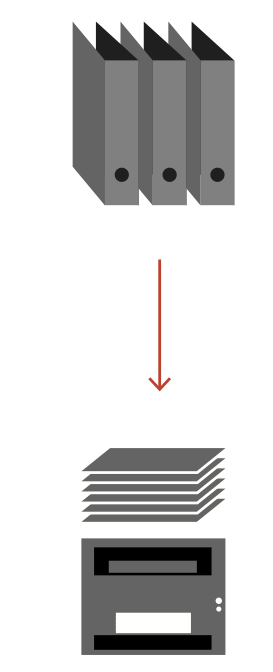
A classic manual archive of patient documents in paper form takes up space, is costly to operate and can only be viewed by stakeholders to a limited extent. The Iterata solution "Scanning for Search & View" immediately creates space that can be used for other purposes and makes the scanned patient documents machine-readable through OCR (Optical Character Recognition) for automated assignment to the patient dossier. The digitized information is immediately searchable and available to all authorized persons. Predefined evaluations and ad-hoc analyses can be immediately extracted from the data in the form of reports and tables.
For the introduction of a scanning solution in a healthcare organization, the focus is on the simple connection of physical and electronic documents to a patient dossier. Our goal is to simplify and automate your process and eliminate unnecessary interfaces. As a unique feature, the system is based on the complete searchability of digital documents (unstructured and structured data) on Iterata's Swiss Digital Health Platform (SDHP).
The search capabilities allow quality checks, migrations, and linking of your data with other medical and administrative content.
As a Digital Health Consortium, we see ourselves as an enabler. Along the phases of access, know-how, new business models, transformation, process efficiencies, education, research and publication, we accompany you as a partner and technology supplier in clinical implementation and digitalization.
Details:
Your benefits and advantages, project planning, modules and price indication
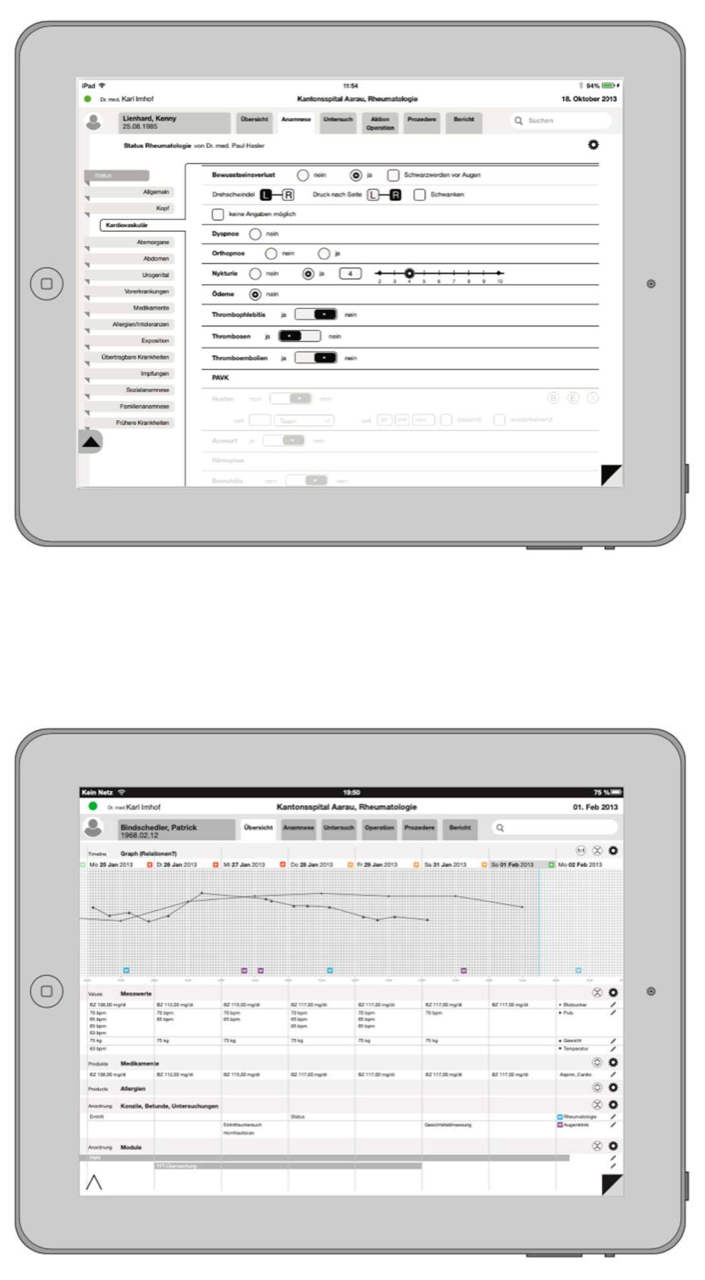
Structured Data Entry
Structured Data Entry (SDE) is a basic prerequisite for an improved decision-making process. Data quality increases, the process is accelerated and costs are optimised. In addition, daily medical practice and research is facilitated by a standardised, clearly formulated and consistent catalogue of questions. If the data entry is structured, the data can be evaluated in real time and there are no longer delays in report generation. The data becomes addressable and therefore searchable and is permanently monitored.
Iterata has developed a solution that allows professionals to define their structures (items) in corresponding lists, trees up to graphs and thus automatically generate their application for data capture in the field of clinical workflow. This allows clinicians to create intelligent applications and forms, including the automatic generation of reports. This tool enables to create personalised and clinician-tailored applications and to build and expand domain knowledge and the corresponding notions of data structures and entities.
Structured Data Entry - comming soon
Link will be provided: Your benefits and advantages with Structured Data Entry
Diagnostic Profiles and Monitoring
- Diagnostic Search Profiles - comming soon
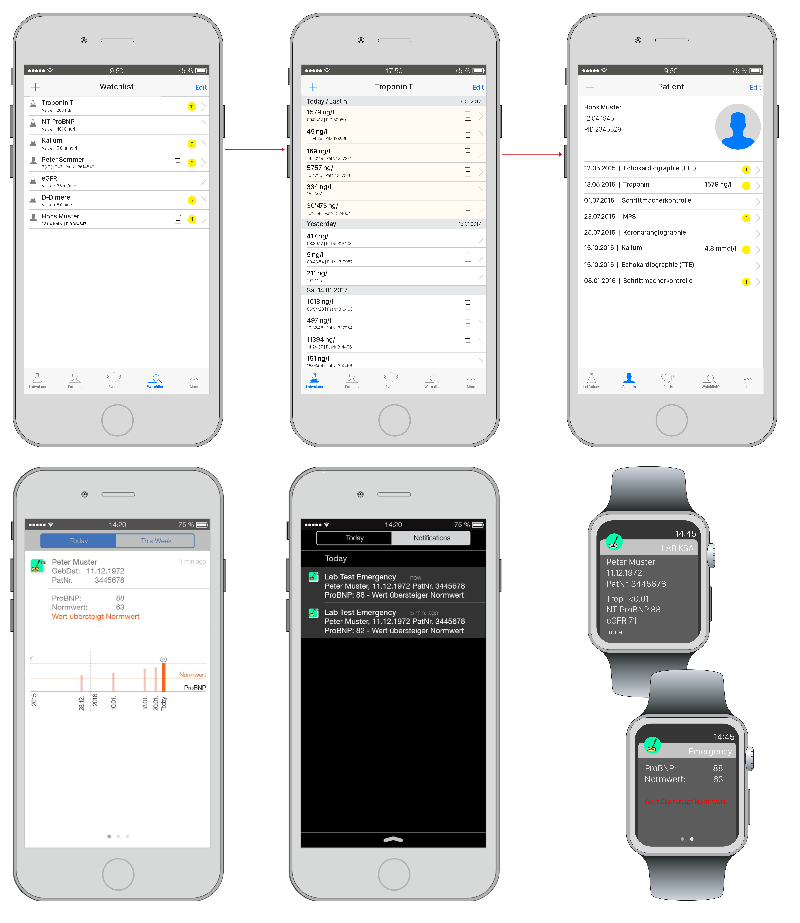
Speed
- Life Saving Apps for Laboratories and Clinics (e.g. Cardiology)
- Speed Apps for clinical decision support
- Escalation & Notification Application for critical or pathological lab value
- Screening of high risk patients through search profiles & algorithms
Quality
- Increase your Master Data Quality
- Curate your Data, Outcomes and Results
- Data protection Solutions based on GDPR guidelines